Heterotetramer
It has been suggested that this article be merged into Tetramer. (Discuss) Proposed since August 2018. |
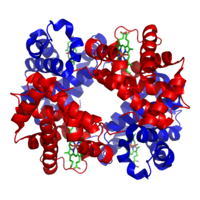
The heterotetrameric molecule haemoglobin, made up of four subunits of two different types (coloured red and blue.)
A heterotetramer is protein containing four non-covalently bound subunits, wherein the subunits are not all identical.[1] A homotetramer contains four identical subunits.[2]
Examples include haemoglobin (pictured), the NMDA receptor, some aquaporins,[3] some AMPA receptors, as well as some enzymes.[4]
Purification of heterotetramers
Ion-exchange chromatography is useful for isolating specific heterotetrameric protein assemblies, allowing purification of specific complexes according to both the number and the position of charged peptide tags.[5][6]Nickel affinity chromatography may also be employed for heterotetramer purification.[7]
See also
- Tetrameric protein
References
^ "GO term: protein heterotetramerization". YeastGenome. Archived from the original on 27 September 2011. Retrieved 14 May 2011..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "GO term: protein homotetramerization". YeastGenome. Archived from the original on 27 September 2011. Retrieved 14 May 2011.
^ Neely, John D.; Christensen, Birgitte M.; Nielsen, Søren; Agre, Peter (1 August 1999). "Heterotetrameric Composition of Aquaporin-4 Water Channels". Biochemistry. 38 (34): 11156–63. doi:10.1021/bi990941s. PMID 10460172.
^ Chang, T.-H.; Hsieh, F.-L.; Ko, T.-P.; Teng, K.-H.; Liang, P.-H.; Wang, A. H.-J. (5 February 2010). "Structure of a Heterotetrameric Geranyl Pyrophosphate Synthase from Mint (Mentha piperita) Reveals Intersubunit Regulation". Plant Cell. 22 (2): 454–467. doi:10.1105/tpc.109.071738. PMC 2845413. PMID 20139160.
^ Sakash, J.B.; Kantrowitz, E.R. (2000). "The contribution of individual interchain interactions to the stabilization of the T and R states of Escherichia coli aspartate transcarbamoylase". J Biol Chem. 275 (37): 28701–7. doi:10.1074/jbc.M005079200. PMID 10875936.
^ Fairhead, M. (2013). "Plug-and-Play Pairing via Defined Divalent Streptavidins". J Mol Biol. 426 (1): 199–214. doi:10.1016/j.jmb.2013.09.016. PMC 4047826. PMID 24056174.
^ Howarth, Mark; Chinnapen, Daniel J-F; Gerrow, Kimberly; Dorrestein, Pieter C; Grandy, Melanie R; Kelleher, Neil L; El-Husseini, Alaa; Ting, Alice Y (2006). "A monovalent streptavidin with a single femtomolar biotin binding site". Nature Methods. 3 (4): 267–73. doi:10.1038/nmeth861. PMC 2576293. PMID 16554831.
This protein-related article is a stub. You can help Wikipedia by expanding it. |