Amine
| Primary (1°) amine | Secondary (2°) amine | Tertiary (3°) amine |
|---|---|---|
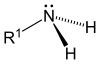 |  | 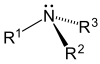 |
In organic chemistry, amines (/əˈmiːn, ˈæmiːn/,[1][2]UK also /ˈeɪmiːn/)[3] are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group[4] (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as chloramine (NClH2); see Category:Inorganic amines.[5]
The substituent -NH2 is called an amino group.[6]
Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure R–CO–NR′R″, are called amides and have different chemical properties from amines.
Contents
1 Classification of amines
2 Naming conventions
3 Physical properties
3.1 Spectroscopic identification
4 Structure
4.1 Alkyl amines
4.2 Aromatic amines
5 Basicity
5.1 Electronic effects
5.2 Solvation effects
6 Synthesis
6.1 Alkylation
6.2 Reductive routes
6.3 Specialized methods
7 Reactions
7.1 Alkylation, acylation, and sulfonation
7.2 Diazotization
7.3 Conversion to imines
7.4 Overview
8 Biological activity
9 Application of amines
9.1 Dyes
9.2 Drugs
9.3 Gas treatment
10 Safety
11 See also
12 References
13 External links
Classification of amines
Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic amines have the nitrogen atom connected to an aromatic ring.
Amines, alkyl and aryl alike, are organized into three subcategories (see table) based on the number of carbon atoms adjacent to the nitrogen:[6]
Primary (1°) amines—Primary amines arise when one of three hydrogen atoms in ammonia is replaced by an alkyl or aromatic group. Important primary alkyl amines include, methylamine, most amino acids, and the buffering agent tris, while primary aromatic amines include aniline.
Secondary (2°) amines—Secondary amines have two organic substituents (alkyl, aryl or both) bound to the nitrogen together with one hydrogen. Important representatives include dimethylamine, while an example of an aromatic amine would be diphenylamine.
Tertiary (3°) amines—In tertiary amines, nitrogen has three organic substituents. Examples include trimethylamine, which has a distinctively fishy smell, and EDTA.
A fourth subcategory is determined by the connectivity of the substituents attached to the nitrogen:
Cyclic amines—Cyclic amines are either secondary or tertiary amines. Examples of cyclic amines include the 3-membered ring aziridine and the six-membered ring piperidine. N-methylpiperidine and N-phenylpiperidine are examples of cyclic tertiary amines.
It is also possible to have four organic substituents on the nitrogen. These species are not amines but are quaternary ammonium cations and have a charged nitrogen center. Quaternary ammonium salts exist with many kinds of anions.
Naming conventions
Amines are named in several ways. Typically, the compound is given the prefix "amino-" or the suffix: "-amine". The prefix "N-" shows substitution on the nitrogen atom. An organic compound with multiple amino groups is called a diamine, triamine, tetraamine and so forth.
Systematic names for some common amines:
| Lower amines are named with the suffix -amine.
| Higher amines have the prefix amino as a functional group. IUPAC however does not recommend this convention,[citation needed] but prefers the alkanamine form, e.g. pentan-2-amine.
|
Physical properties
Hydrogen bonding significantly influences the properties of primary and secondary amines. For example, methyl and ethyl amines are gases under standard conditions, whereas the corresponding methyl and ethyl alcohols are liquids. Amines possess a characteristic ammonia smell, liquid amines have a distinctive "fishy" smell.
The nitrogen atom features a lone electron pair that can bind H+ to form an ammonium ion R3NH+. The lone electron pair is represented in this article by a two dots above or next to the N. The water solubility of simple amines is enhanced by hydrogen bonding involving these lone electron pairs. Typically salts of ammonium compounds exhibit the following order of solubility in water: primary ammonium (RNH+
3) > secondary ammonium (R
2NH+
2) > tertiary ammonium (R3NH+). Small aliphatic amines display significant solubility in many solvents, whereas those with large substituents are lipophilic. Aromatic amines, such as aniline, have their lone pair electrons conjugated into the benzene ring, thus their tendency to engage in hydrogen bonding is diminished. Their boiling points are high and their solubility in water is low.
Spectroscopic identification
Typically the presence of an amine functional group is deduced by a combination of techniques, including mass spectrometry as well as NMR and IR spectroscopies. 1H NMR signals for amines disappear upon treatment of the sample with D2O. In their infrared spectrum primary amines exhibit two N-H bands, whereas secondary amines exhibit only one.[6]
Structure
Alkyl amines
Alkyl amines characteristically feature tetrahedral nitrogen centers. C-N-C and C-N-H angles approach the idealized angle of 109°. C-N distances are slightly shorter than C-C distances. The energy barrier for the nitrogen inversion of the stereocenter is about 7 kcal/mol for a trialkylamine. The interconversion has been compared to the inversion of an open umbrella into a strong wind.
Amines of the type NHRR′ and NRR′R″ are chiral: the nitrogen center bears four substituents counting the lone pair. Because of the low barrier to inversion, amines of the type NHRR′ cannot be obtained in optical purity. For chiral tertiary amines, NRR′R″ can only be resolved when the R, R′, and R″ groups are constrained in cyclic structures such as N-substituted aziridines (quaternary ammonium salts are resolvable).
 | ⇌ |  |
| Inversion of an amine. The pair of dots represents the lone electron pair on the nitrogen atom. | ||
Aromatic amines
In aromatic amines ("anilines"), nitrogen is often nearly planar owing to conjugation of the lone pair with the aryl substituent. The C-N distance is correspondingly shorter. In aniline, the C-N distance is the same as the C-C distances.[7]
Basicity
Like ammonia, amines are bases.[8] Compared to alkali metal hydroxides, amines are weaker (see table for examples of conjugate acid Ka values).
| Alkylamine[9] or aniline[10] | pKa of protonated amine | Kb |
|---|---|---|
methylamine (MeNH2) | 10.62 | 4.17E-04 |
dimethylamine (Me2NH) | 10.64 | 4.37E-04 |
trimethylamine (Me3N) | 9.76 | 5.75E-05 |
ethylamine (EtNH2) | 10.63 | 4.27E-04 |
aniline (PhNH2) | 4.62 | 4.17E-10 |
4-methoxyaniline (4-MeOC6H4NH2) | 5.36 | 2.29E-09 |
N,N-Dimethylaniline (PhNMe2) | 5.07 | 1.17E-09 |
3-Nitroaniline (3-NO2-C6H4NH2) | 2.46 | 2.88E-12 |
4-Nitroaniline (4-NO2-C6H4NH2) | 1 | 1.00E-13 |
| 4-trifluoromethylaniline (CF3C6H4NH2) | 2.75 | 5.62E-12 |
The basicity of amines depends on:
- The electronic properties of the substituents (alkyl groups enhance the basicity, aryl groups diminish it).
- The degree of solvation of the protonated amine, which includes steric hindrance by the groups on nitrogen.
Electronic effects
Owing to inductive effects, the basicity of an amine might be expected to increase with the number of alkyl groups on the amine. Correlations are complicated owing to the effects of solvation which are opposite the trends for inductive effects. Solvation effects also dominate the basicity of aromatic amines (anilines). For anilines, the lone pair of electrons on nitrogen delocalises into the ring, resulting in decreased basicity. Substituents on the aromatic ring, and their positions relative to the amine group, also affect basicity as seen in the table.
Solvation effects
Solvation significantly affects the basicity of amines. N-H groups strongly interact with water, especially in ammonium ions. Consequently, the basicity of ammonia is enhanced by 1011 by solvation. The intrinsic basicity of amines, i.e. the situation where solvation is unimportant, has been evaluated in the gas phase. In the gas phase, amines exhibit the basicities predicted from the electron-releasing effects of the organic substituents. Thus tertiary amines are more basic than secondary amines, which are more basic than primary amines, and finally ammonia is least basic. The order of pKb's (basicities in water) does not follow this order. Similarly aniline is more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.[11]
In aprotic polar solvents such as DMSO, DMF, and acetonitrile the energy of solvation is not as high as in protic polar solvents like water and methanol. For this reason, the basicity of amines in these aprotic solvents is almost solely governed by the electronic effects.
Synthesis
Alkylation
The most industrially significant amines are prepared from ammonia by alkylation with alcohols:[5]
- ROH + NH3 → RNH2 + H2O
Unlike the reaction of amines with alkyl halides, the industrial method is green insofar that the coproduct is water. The reaction of amines and ammonia with alkyl halides is used for synthesis in the laboratory:
- RX + 2 R′NH2 → RR′NH + [RR′NH2]X
Such reactions, which are most useful for alkyl iodides and bromides, are rarely employed because the degree of alkylation is difficult to control.[5] Selectivity can be improved via the Delépine reaction, although this is rarely employed on an industrial scale.
Reductive routes
Via the process of hydrogenation, nitriles are reduced to amines using hydrogen in the presence of a nickel catalyst. Reactions are sensitive to acidic or alkaline conditions, which can cause hydrolysis of the –CN group. LiAlH4 is more commonly employed for the reduction of nitriles on the laboratory scale. Similarly, LiAlH4 reduces amides to amines. Many amines are produced from aldehydes and ketones via reductive amination, which can either proceed catalytically or stoichiometrically.
Aniline (C6H5NH2) and its derivatives are prepared by reduction of the nitroaromatics. In industry, hydrogen is the preferred reductant, whereas, in the laboratory, tin and iron are often employed.
Specialized methods
Many laboratory methods exist for the preparation of amines, many of these methods being rather specialized.
| Reaction name | Substrate | Comment |
|---|---|---|
Gabriel synthesis | Organohalide | Reagent: potassium phthalimide |
Staudinger reduction | Azide | This reaction also takes place with a reducing agent such as lithium aluminium hydride. |
Schmidt reaction | Carboxylic acid | |
Aza-Baylis–Hillman reaction | Imine | Synthesis of allylic amines |
Birch reduction | Imine | Useful for reactions that trap unstable imine intermediates, such as Grignard reactions with nitriles.[12] |
Hofmann degradation | Amide | This reaction is valid for preparation of primary amines only. Gives good yields of primary amines uncontaminated with other amines. |
Hofmann elimination | Quaternary ammonium salt | Upon treatment with strong base |
Amide reduction | amide | |
Nitrile reduction | Nitriles | Either accomplished with reducing agents or by electrosynthesis |
Reduction of nitro compounds | Nitro compounds | Can be accomplished with elemental zinc, tin or iron with an acid. |
Amine alkylation | Haloalkane | |
Delepine reaction | Organohalide | reagent Hexamine |
Buchwald–Hartwig reaction | Aryl halide | Specific for aryl amines |
Menshutkin reaction | Tertiary amine | Reaction product a quaternary ammonium cation |
Hydroamination | Alkenes and alkynes | |
Oxime reduction | Oximes | |
Leuckart reaction | Ketones and aldehydes | Reductive amination with formic acid and ammonia via an imine intermediate |
Hofmann–Löffler reaction | Haloamine | |
Eschweiler–Clarke reaction | Amine | Reductive amination with formic acid and formaldehyde via an imine intermediate |
Reactions
Alkylation, acylation, and sulfonation
Aside from their basicity, the dominant reactivity of amines is their nucleophilicity.[13] Most primary amines are good ligands for metal ions to give coordination complexes. Amines are alkylated by alkyl halides. Acyl chlorides and acid anhydrides react with primary and secondary amines to form amides (the "Schotten–Baumann reaction").
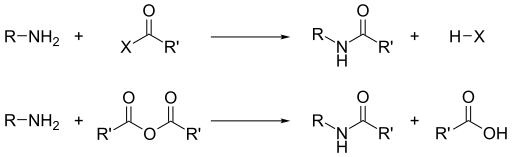
Similarly, with sulfonyl chlorides, one obtains sulfonamides. This transformation, known as the Hinsberg reaction, is a chemical test for the presence of amines.
Because amines are basic, they neutralize acids to form the corresponding ammonium salts R3NH+. When formed from carboxylic acids and primary and secondary amines, these salts thermally dehydrate to form the corresponding amides.
- H−N|R2R1|:⏟amine+R3−CO‖−OH⏟carboxylic acid−> H−N+|R2R1|−H+R3−COO−⏟substituted-ammoniumcarboxylate salt→dehydrationheatN|R2R1|−CO‖−R3⏟amide+H2O⏟water{displaystyle {underbrace {ce {H-!!{overset {displaystyle R1 atop |}{underset {| atop displaystyle R2}{N}}}!!!!:}} _{amine}+underbrace {ce {R3-{overset {displaystyle O atop |}{C}}-OH}} _{text{carboxylic acid}}->} underbrace {ce {{H-{overset {displaystyle R1 atop |}{underset {| atop displaystyle R2}{N+}}}-H}+R3-COO^{-}}} _{{text{substituted-ammonium}} atop {text{carboxylate salt}}}{ce {->[heat][dehydration]}}{underbrace {ce {{overset {displaystyle R1 atop |}{underset {| atop displaystyle R2}{N}}}!!-{overset {displaystyle O atop |}{C}}-R3}} _{amide}+underbrace {ce {H2O}} _{water}}}
Diazotization
Amines react with nitrous acid to give diazonium salts. The alkyl diazonium salts are of little synthetic importance because they are too unstable. The most important members are derivatives of aromatic amines such as aniline ("phenylamine") (A = aryl or naphthyl):
- ANH2+HNO2+HX⟶AN+2X−+2H2O{displaystyle {ce {ANH2 + HNO2 + HX -> AN + 2X^- + 2 H2O}}}
Anilines and naphthylamines form more stable diazonium salts, which can be isolated in the crystalline form.[14] Diazonium salts undergo a variety of useful transformations involving replacement of the N2 group with anions. For example, cuprous cyanide gives the corresponding nitriles:
- AN2++Y−⟶AY+N2{displaystyle {ce {AN2+ + Y^- -> AY + N2}}}
Aryldiazonium couple with electron-rich aromatic compounds such as a phenol to form azo compounds. Such reactions are widely applied to the production of dyes.[15]
Conversion to imines
Imine formation is an important reaction. Primary amines react with ketones and aldehydes to form imines. In the case of formaldehyde (R′ = H), these products typically exist as cyclic trimers.
- RNH2 + R′2C=O → R′2C=NR + H2O
Reduction of these imines gives secondary amines:
- R′2C=NR + H2 → R′2CH–NHR
Similarly, secondary amines react with ketones and aldehydes to form enamines:
- R2NH + R′(R″CH2)C=O → R″CH=C(NR2)R′ + H2O
Overview
An overview of the reactions of amines is given below:
| Reaction name | Reaction product | Comment |
|---|---|---|
Amine alkylation | Amines | Degree of substitution increases |
Schotten–Baumann reaction | Amide | Reagents: acyl chlorides, acid anhydrides |
Hinsberg reaction | Sulfonamides | Reagents: sulfonyl chlorides |
Amine–carbonyl condensation | Imines | |
Organic oxidation | Nitroso compounds | Reagent: peroxymonosulfuric acid |
Organic oxidation | Diazonium salt | Reagent: nitrous acid |
Zincke reaction | Zincke aldehyde | Reagent: pyridinium salts, with primary and secondary amines |
Emde degradation | Tertiary amine | Reduction of quaternary ammonium cations |
Hofmann–Martius rearrangement | Aryl-substituted anilines | |
von Braun reaction | Organocyanamide | By cleavage (tertiary amines only) with cyanogen bromide |
Hofmann elimination | Alkene | Proceeds by β-elimination of less hindered carbon |
Cope reaction | Alkene | Similar to Hofmann elimination |
carbylamine reaction | Isonitrile | Primary amines only |
| Hoffmann's mustard oil test | Isothiocyanate | CS2 and HgCl2 are used. Thiocyanate smells like mustard. |
Biological activity
Amines are ubiquitous in biology. The breakdown of amino acids releases amines, famously in the case of decaying fish which smell of trimethylamine. Many neurotransmitters are amines, including epinephrine, norepinephrine, dopamine, serotonin, and histamine. Protonated amino groups (–NH+
3) are the most common positively charged moieties in proteins, specifically in the amino acid lysine.[16] The anionic polymer DNA is typically bound to various amine-rich proteins.[17] Additionally, the terminal charged primary ammonium on lysine forms salt bridges with carboxylate groups of other amino acids in polypeptides, which is one of the primary influences on the three-dimensional structures of proteins.[18]
Application of amines
Dyes
Primary aromatic amines are used as a starting material for the manufacture of azo dyes. It reacts with nitrous acid to form diazonium salt, which can undergo coupling reaction to form an azo compound. As azo-compounds are highly coloured, they are widely used in dyeing industries, such as:
- Methyl orange
- Direct brown 138
Sunset yellow FCF- Ponceau
Drugs
Many drugs are designed to mimic or to interfere with the action of natural amine neurotransmitters, exemplified by the amine drugs:
Chlorpheniramine is an antihistamine that helps to relieve allergic disorders due to cold, hay fever, itchy skin, insect bites and stings.
Chlorpromazine is a tranquilizer that sedates without inducing sleep. It is used to relieve anxiety, excitement, restlessness or even mental disorder.
Ephedrine and phenylephrine, as amine hydrochlorides, are used as decongestants.
Amphetamine, methamphetamine, and methcathinone are psychostimulant amines that are listed as controlled substances by the US DEA.
Amitriptyline, imipramine, lofepramine and clomipramine are tricyclic antidepressants and tertiary amines.
Nortriptyline, desipramine, and amoxapine are tricyclic antidepressants and secondary amines. (The tricyclics are grouped by the nature of the final amine group on the side chain.)
Substituted tryptamines and phenethylamines are key basic structures for a large variety of psychedelic drugs.
Opiate analgesics such as morphine, codeine, and heroin are tertiary amines.
Gas treatment
Aqueous monoethanolamine (MEA), diglycolamine (DGA), diethanolamine (DEA), diisopropanolamine (DIPA) and methyldiethanolamine (MDEA) are widely used industrially for removing carbon dioxide (CO2) and hydrogen sulfide (H2S) from natural gas and refinery process streams. They may also be used to remove CO2 from combustion gases and flue gases and may have potential for abatement of greenhouse gases. Related processes are known as sweetening.[19]
Safety
Low molecular weight simple amines, such as ethylamine, are only weakly toxic with LD50 between 100 and 1000 mg/kg. They are skin irritants, especially as some are easily absorbed through the skin.[5] Amines are a broad class of compounds, and more complex members of the class can be extremely bioactive, for example strychnine and heroin.
See also
- Acid-base extraction
- Amine gas treating
- Ammine
- Biogenic amine
- Imine
IUPAC nomenclature for the official naming rules for amines.- Ligand isomerism
References
^ "amine". The American Heritage Dictionary of the English Language (5th ed.). Boston: Houghton Mifflin Harcourt. 2014..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "Amine definition and meaning". Collins English Dictionary. Retrieved 2017-03-28.
^ "amine - definition of amine in English". Oxford Dictionaries. Retrieved 2017-03-28.
^ McMurry, John E. (1992), Organic Chemistry (3rd ed.), Belmont: Wadsworth, ISBN 0-534-16218-5
^ abcd Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a02_001. ISBN 3527306730.
^ abc Smith, Janice Gorzynski (2011). "Chapter 25 Amines". Organic chemistry (Book) (3rd ed.). New York, NY: McGraw-Hill. pp. 949–993. ISBN 978-0-07-337562-5.
^ G. M. Wójcik "Structural Chemistry of Anilines" in Anilines (Patai's Chemistry of Functional Groups), S. Patai, Ed. 2007, Wiley-VCH, Weinheim. doi:10.1002/9780470682531.pat0385
^ J. W. Smith (1968). S. Patai, ed. "Basicity and complex formation". Patai's Chemistry of Functional Groups. doi:10.1002/9780470771082.ch4.
^ Hall, H. K. (1957). "Correlation of the Base Strengths of Amines". Journal of the American Chemical Society. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
^ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". The Journal of Organic Chemistry. 70 (3): 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7
^ Weiberth, Franz J.; Hall, Stan S. (1986). "Tandem alkylation-reduction of nitriles. Synthesis of branched primary amines". Journal of Organic Chemistry. 51 (26): 5338–5341. doi:10.1021/jo00376a053.
^ March, Jerry (1992), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (4th ed.), New York: Wiley, ISBN 0-471-60180-2
^ A. N. Nesmajanow (1943). "β-Naphthylmercuric chloride". Organic Syntheses.; Collective Volume, 2, p. 432
^ Hunger, Klaus; Mischke, Peter; Rieper, Wolfgang; Raue, Roderich; Kunde, Klaus; Engel, Aloys (2000). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a03_245. ISBN 3527306730.
^ Andrade, Miguel A.; O'Donoghue, Seán I.; Rost, Burkhard (1998). "Adaptation of protein surfaces to subcellular location". Journal of Molecular Biology. 276 (2): 517–25. CiteSeerX 10.1.1.32.3499. doi:10.1006/jmbi.1997.1498. PMID 9512720.
^ Nelson, D. L.; Cox, M. M. (2000). Lehninger, Principles of Biochemistry (3rd ed.). New York: Worth Publishing. ISBN 1-57259-153-6.
^ Dill, Ken A. (1990). "Dominant forces in protein folding". Biochemistry. 29 (31): 7133–55. doi:10.1021/bi00483a001. PMID 2207096.
^ Hammer, Georg; Lübcke, Torsten; Kettner, Roland; Davis, Robert N.; Recknagel, Herta; Commichau, Axel; Neumann, Hans-Joachim; Paczynska-Lahme, Barbara (2000). "Natural Gas". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a17_073. ISBN 3527306730.
External links
| Wikiquote has quotations related to: Amine |
Primary amine synthesis: synthetic protocols from organic-reaction.com
Synthesis of amines from organic-chemistry.org
[1] Amines have been implicated in migraine headaches; link contains citations, and list of amine containing foods.

![Amine reaction with carboxylic acids {displaystyle {underbrace {ce {H-!!{overset {displaystyle R1 atop |}{underset {| atop displaystyle R2}{N}}}!!!!:}} _{amine}+underbrace {ce {R3-{overset {displaystyle O atop |}{C}}-OH}} _{text{carboxylic acid}}->} underbrace {ce {{H-{overset {displaystyle R1 atop |}{underset {| atop displaystyle R2}{N+}}}-H}+R3-COO^{-}}} _{{text{substituted-ammonium}} atop {text{carboxylate salt}}}{ce {->[heat][dehydration]}}{underbrace {ce {{overset {displaystyle R1 atop |}{underset {| atop displaystyle R2}{N}}}!!-{overset {displaystyle O atop |}{C}}-R3}} _{amide}+underbrace {ce {H2O}} _{water}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ed9e4ee62efb585271572cbf0fd9149c90a400fd)

